The European Board and College of Obstetrics and Gynaecology (EBCOG) and Flo Health Inc., the developer of Flo, an AI-powered women’s health app, have signed a cooperation agreement.
-
Tracking cycle
-
Getting pregnant
-
Pregnancy
-
Help Center
-
Flo for Partners
-
Anonymous Mode
-
Flo app reviews
-
Flo Premium New
-
Secret Chats New
-
Symptom Checker New
-
Your cycle
-
Health 360°
-
Getting pregnant
-
Pregnancy
-
Being a mom
-
LGBTQ+
-
Quizzes
-
Ovulation calculator
-
hCG calculator
-
Pregnancy test calculator
-
Menstrual cycle calculator
-
Period calculator
-
Implantation calculator
-
Pregnancy weeks to months calculator
-
Pregnancy due date calculator
-
IVF and FET due date calculator
-
Due date calculator by ultrasound
-
Medical Affairs
-
Science & Research
-
Pass It On Project New
-
Privacy Portal
-
Press Center
-
Flo Accuracy
-
Careers
-
Contact Us
Flo Signs Cooperation Agreement with EBCOG


April 17, 2019
Under the terms of the agreement, experts from EBCOG and Flo will jointly develop educational content on a wide range of medical topics. Both parties believe that this cooperation will contribute to raising global awareness about female health. Flo aims to provide millions of users across the globe with evidence-based medical information. The partnership with EBCOG, a leading medical organization and thought leader in obstetrics and gynecology across the EU, will elevate the standards of information women receive every day in their Flo app.
"Allowing easier access to increasingly more accurate information on topics related to globalwomen’s health will be the main goal of this cooperation. EBCOG and Flo will excel in achieving this combination of facts. This partnership with Flo is, in fact, a perfect example of what the aims and objectives of EBCOG are, as stated in the 2nd article of its Constitution: to improve the health of women and their babies by seeking to achieve the highest possible standards of training and care in the field of obstetrics and gynecology,” EBCOG’s Secretary-General Nuno Martins said.

Allowing easier access to increasingly more accurate information on topics related to globalwomen’s health will be the main goal of this cooperation.
"The delivery of credible best-in-class content for our users is paramount for Flo. Flo users highly value the app’s Health Insights component. Content in Flo is personalized, i.e. users receive daily articles and videos based on their goals and the body signals they log and track. The partnership with EBCOG is incredibly important for us because it will allow our users to receive the most credible information based on the most recent scientific findings. With Flo and EBCOG’s joint efforts, we can make sure we deliver this information to the right people at the right time, allowing us to help people in the most effective way possible,” Flo Chief Science Officer Anna Klepchukova said.
Take a quiz
Find out what you can do with our Health Assistant

We aim to provide a significant benefit to women’s health and believe it can be delivered through the power of joint efforts.
"That is why we are cooperating with EBCOG, the leading medical organization in the EU that aims to improve the health of women and their babies, to provide our users with the most relevant and useful information based on the latest scientific achievements and thought leadership.
In addition to cooperating on content creation, Flo Health will support EBCOG events and scientific projects of EBCOG members, which is a great opportunity for us to enhance our experience and share our learnings,” Anna Klepchukova added.
About EBCOG
EBCOG is the Board of the Section of Gynaecology and Obstetrics of the Union Européenne des Médecins Spécialistes (UEMS).
EBCOG has 4 Officers (Professor Jacky Nizard - President; Dr. Nuno Martins - Secretary-General; Professor Basil Tarlatzis - Vice President; and Professor Diethelm Wallwiener - Treasurer), an Executive Committee (including 5 Executive members representing Regions; the SCTA - Standing Committee on Training and Assessment; SCTR – Standing Committee on Training Recognition; SCE – Standing Committee on Examinations; ENTOG – the European Network of Trainees in Obstetrics and Gynaecology; 3 Working Groups – Position Statements, Congress, and Fellowships), and a Council made up of two national delegates from each of our 34 member countries.
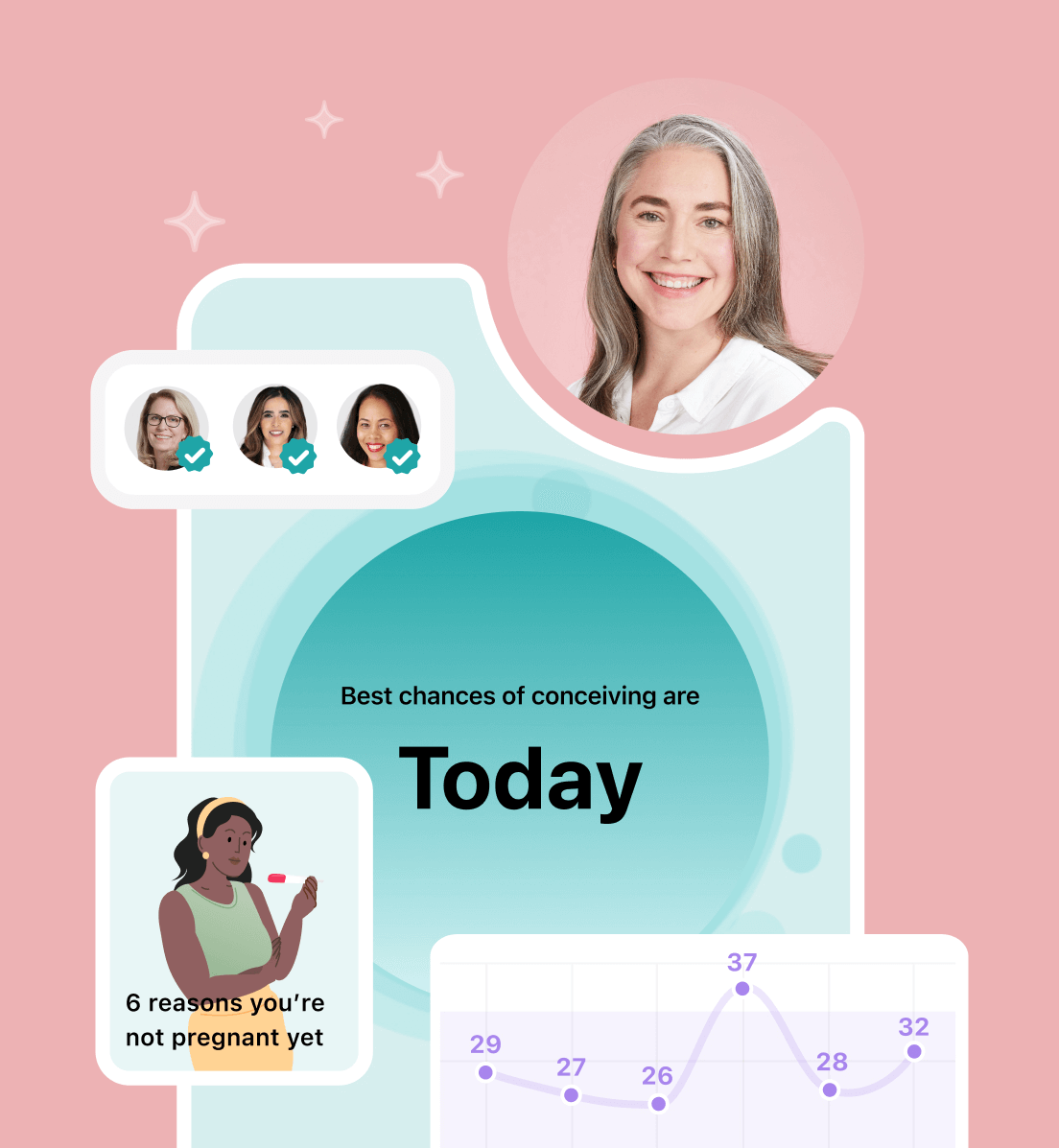
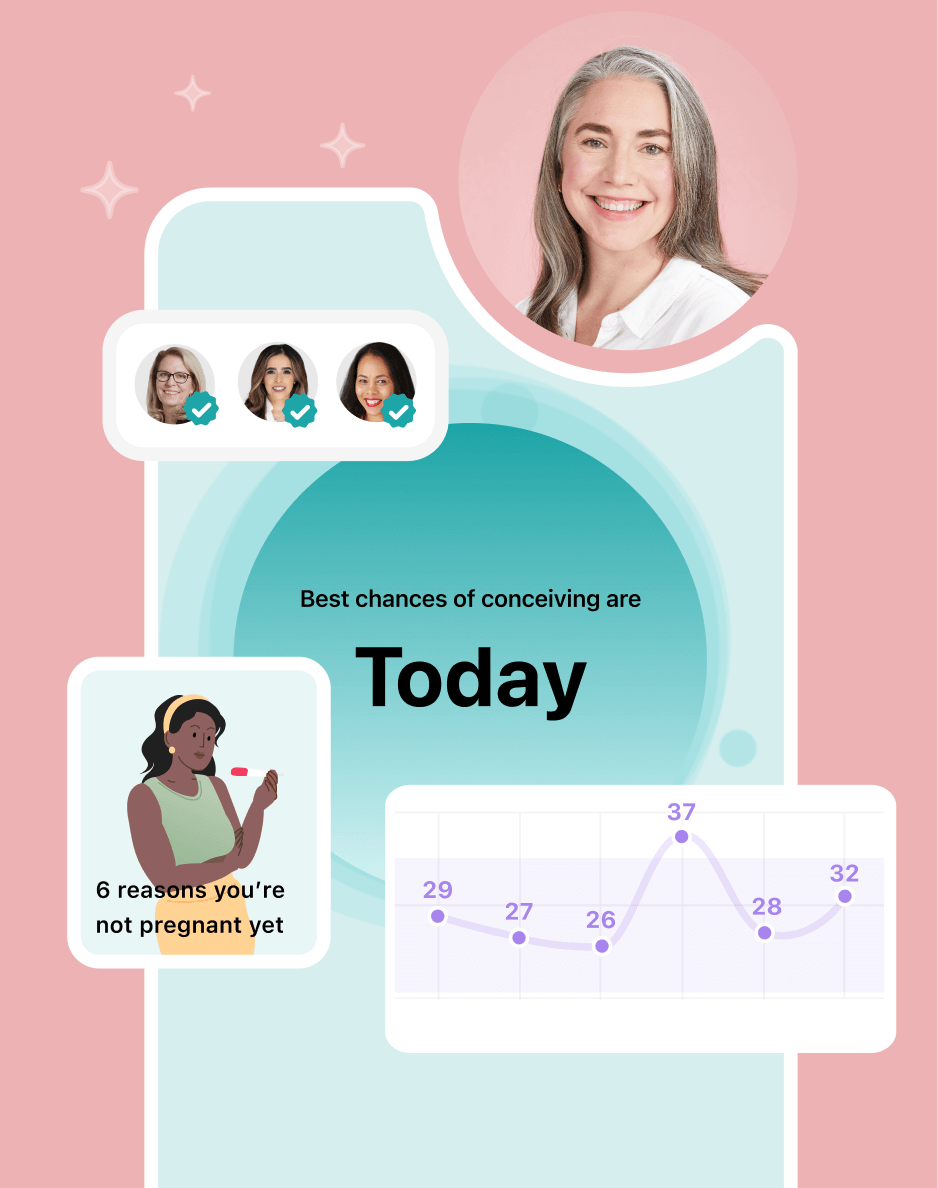
Hey, I'm Anique
I started using Flo app to track my period and ovulation because we wanted to have a baby.
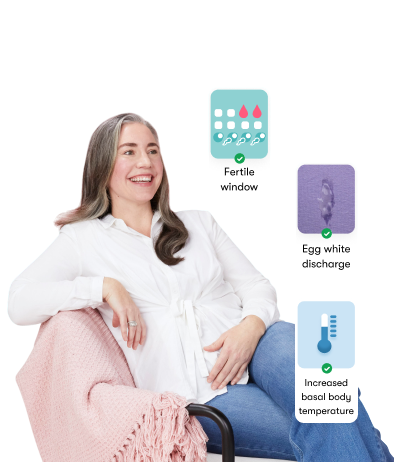
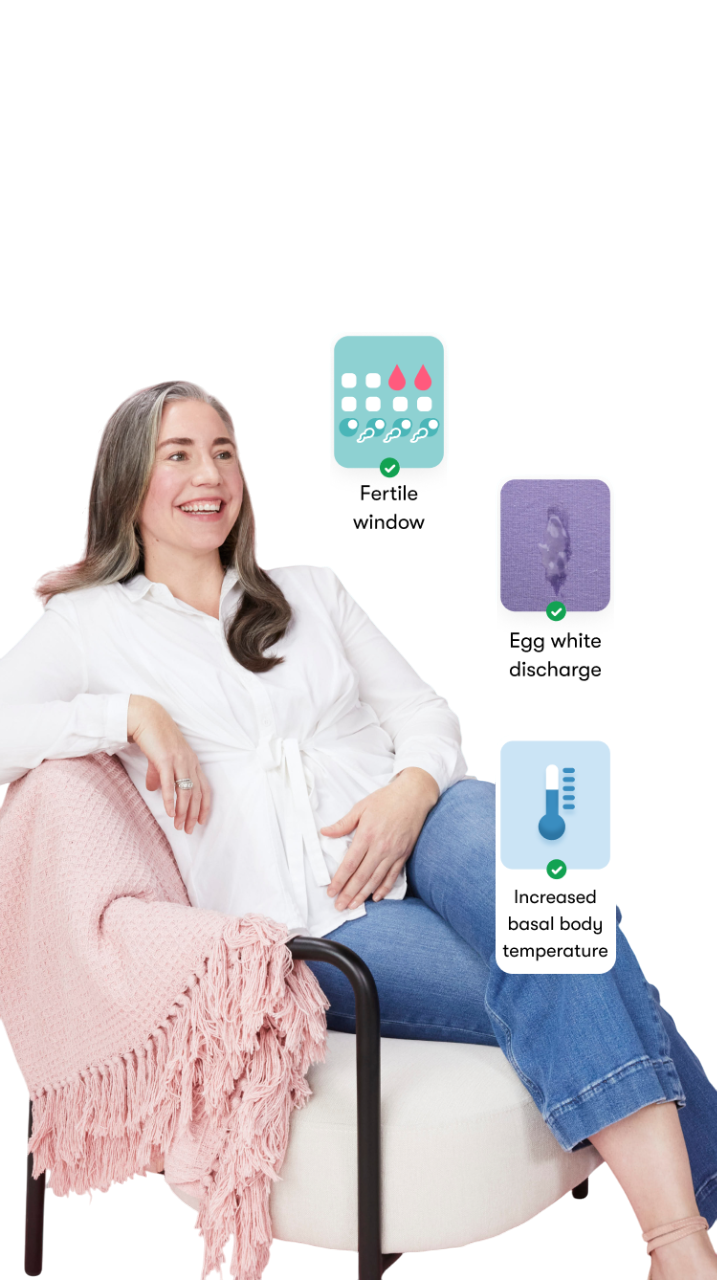
The Flo app helped me learn about my body and spot ovulation signs during our conception journey.


I vividly
remember the day
that we switched
Flo into
Pregnancy Mode — it was
such a special
moment.
Real stories, real results
Learn how the Flo app became an amazing cheerleader for us on our conception journey.




